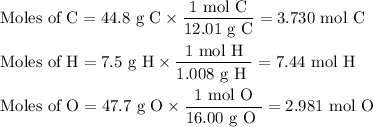Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3


Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
The sugar deoxyribose is an important component of DNA. Deoxyribose is 44.8% 7.5% H, and 47.7% O by...
Questions

Mathematics, 24.05.2020 00:02

Mathematics, 24.05.2020 00:02

Mathematics, 24.05.2020 00:02






Computers and Technology, 24.05.2020 00:02




Mathematics, 24.05.2020 00:02


Mathematics, 24.05.2020 00:02



English, 24.05.2020 00:02

Mathematics, 24.05.2020 00:02

History, 24.05.2020 00:02