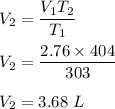Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3


Chemistry, 23.06.2019 09:00
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground,its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
A sample of a gas occupies 2.76 L at 303K. What would the volume be if the temperature was increased...
Questions

Mathematics, 05.05.2020 22:02


History, 05.05.2020 22:02


History, 05.05.2020 22:02

Chemistry, 05.05.2020 22:02

Mathematics, 05.05.2020 22:02


Physics, 05.05.2020 22:02

Mathematics, 05.05.2020 22:02

Mathematics, 05.05.2020 22:02


Mathematics, 05.05.2020 22:02


Mathematics, 05.05.2020 22:02



Physics, 05.05.2020 22:02


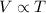
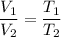
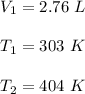
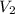 is new volume. Using above formula we get :
is new volume. Using above formula we get :