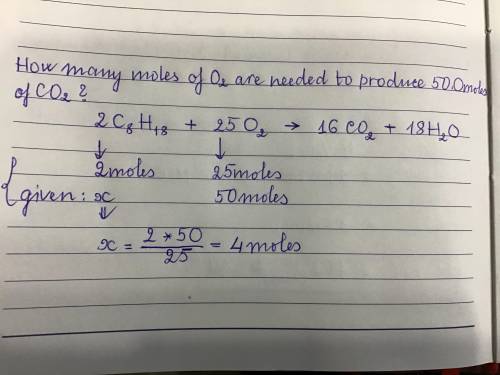How many moles of O2 are needed to produce 50.0 moles of CO2?
2 C8H18 + 25 O2 --> 16 CO2 +...

Chemistry, 21.05.2020 00:12 sandygarcia65
How many moles of O2 are needed to produce 50.0 moles of CO2?
2 C8H18 + 25 O2 --> 16 CO2 + 18 H20
a) 78.1 moles

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 23.06.2019 14:20
Identificaa 5 características que comparten las guacamayas y dos caracteristicas que las hagan diferentes
Answers: 1

Chemistry, 23.06.2019 15:30
In most resting cells, the concentration of sodium ions is higher outside of cells compared with the intracellular fluid. when cells are stimulated, sodium ion channels open, and sodium diffuses from the outside of the cell to the inside of the cell. sodium ion concentrations in a resting cell are an example of and sodium ion movement in a stimulated cell is an example of in most resting cells, the concentration of sodium ions is higher outside of cells compared with the intracellular fluid. when cells are stimulated, sodium ion channels open, and sodium diffuses from the outside of the cell to the inside of the cell. sodium ion concentrations in a resting cell are an example of and sodium ion movement in a stimulated cell is an example of potential energy; kinetic energy kinetic energy; potential energy the energy of motion; stored energy chemical work; energy stored in chemical bonds
Answers: 2
You know the right answer?
Questions



Mathematics, 20.11.2020 07:30



Physics, 20.11.2020 07:30

History, 20.11.2020 07:30





Mathematics, 20.11.2020 07:30



Arts, 20.11.2020 07:30


Mathematics, 20.11.2020 07:30


English, 20.11.2020 07:30