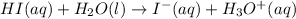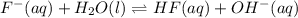Chemistry, 21.05.2020 00:07 Christyy9608
Identify the reactant that is a Brønsted−Lowry acid in the following reaction: HI(aq)+H2O(l)→I−(aq)+H3O+(aq) Express your answer as a chemical formula. nothing Request Answer Part B Identify the reactant that is a Brønsted−Lowry base in the following reaction: HI(aq)+H2O(l)→I−(aq)+H3O+(aq) Express your answer as a chemical formula. nothing Request Answer Part C Identify the reactant that is a Brønsted−Lowry acid in the following reaction: F−(aq)+H2O(l)⇌HF(aq)+OH−(aq) Express your answer as a chemical formula. nothing Request Answer Part D Identify the reactant that is a Brønsted−Lowry base in the following reaction: F−(aq)+H2O(l)⇌HF(aq)+OH−(aq) Express your answer as a chemical formula.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Identify the reactant that is a Brønsted−Lowry acid in the following reaction: HI(aq)+H2O(l)→I−(aq)+...
Questions





Biology, 05.07.2019 14:20


Mathematics, 05.07.2019 14:20

Biology, 05.07.2019 14:20

Chemistry, 05.07.2019 14:20

English, 05.07.2019 14:20


History, 05.07.2019 14:20

History, 05.07.2019 14:20

History, 05.07.2019 14:20


World Languages, 05.07.2019 14:20

English, 05.07.2019 14:20


History, 05.07.2019 14:20

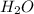 is Bronsted-Lowry base.
is Bronsted-Lowry base.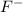 is Bronsted-Lowry base.
is Bronsted-Lowry base.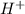 ).
).