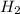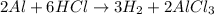Chemistry, 19.05.2020 15:13 imamador6396
Identify the product(s) in the chemical equation below. Check all that apply.
Al + 6 HCI - 3 H2 + 2 AICI:
H2
HCI
AI
AICI3

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3
You know the right answer?
Identify the product(s) in the chemical equation below. Check all that apply.
Al + 6 HCI - 3 H...
Al + 6 HCI - 3 H...
Questions



Mathematics, 20.05.2021 02:10


Mathematics, 20.05.2021 02:10



Chemistry, 20.05.2021 02:10



History, 20.05.2021 02:10




Mathematics, 20.05.2021 02:10

Mathematics, 20.05.2021 02:10

Mathematics, 20.05.2021 02:10



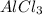 and
and