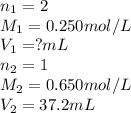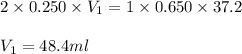Chemistry, 19.05.2020 03:15 evanwall91
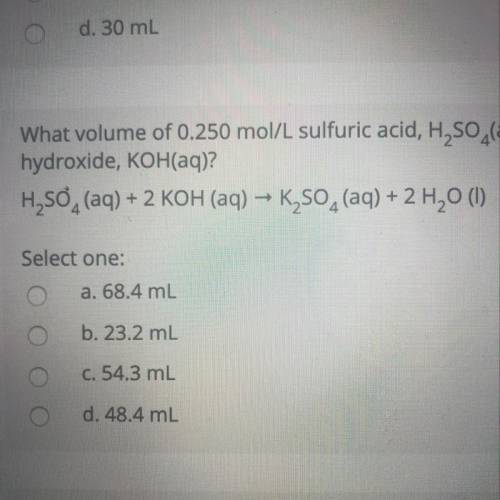
What volume of 0.250 mol/L sulfuric acid, H2SO4(aq) is needed to react completely with 37.2 mL of 0.650 mol/L potassium hydroxide, KOH(aq)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which statement best describes how atoms combine to form sodium chloride (nacl)? a. a positively charged sodium ion and a positively charged chlorine ion form an covalent bond. b. a positively charged sodium ion and a negatively charged chlorine ion form an covalent bond. c. a positively charged sodium ion and a positively charged chlorine ion form an ionic bond. d. a positively charged sodium ion and a negatively charged chlorine ion form an ionic bond.
Answers: 1


Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
What volume of 0.250 mol/L sulfuric acid, H2SO4(aq) is needed to react completely with 37.2 mL of 0....
Questions


Mathematics, 21.05.2020 02:59

Law, 21.05.2020 02:59



Mathematics, 21.05.2020 02:59

Mathematics, 21.05.2020 02:59


Mathematics, 21.05.2020 02:59




Mathematics, 21.05.2020 02:59


Mathematics, 21.05.2020 02:59





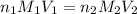
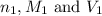 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 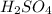
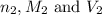 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.