
Chemistry, 19.05.2020 03:17 Redhead667
1.What mass of oxygen gas, O2, from the air is consumed in the combustion of 702g of octane, C8H18, one of the principal components of gasoline? 2C8H18 + 25O2 -> 16CO2 + 18H2O.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

You know the right answer?
1.What mass of oxygen gas, O2, from the air is consumed in the combustion of 702g of octane, C8H18,...
Questions


Arts, 31.01.2020 13:46





Chemistry, 31.01.2020 13:46

Mathematics, 31.01.2020 13:46


Mathematics, 31.01.2020 13:46


History, 31.01.2020 13:46


Mathematics, 31.01.2020 13:46


Biology, 31.01.2020 13:46

History, 31.01.2020 13:47


English, 31.01.2020 13:47

Mathematics, 31.01.2020 13:47

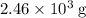 .
.  :
: 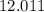 .
. :
: 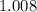 .
. :
: 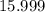 .
.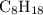 :
: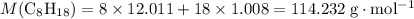 .
.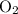 :
: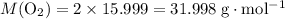 .
.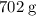 of octane,
of octane, 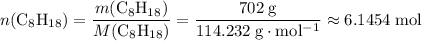 .
.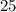 , whileThe coefficient of
, whileThe coefficient of 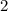 .
.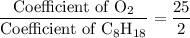 .
.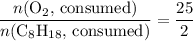 .
.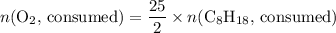 .
.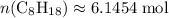 . Hence, the number of moles of oxygen gas required will be:
. Hence, the number of moles of oxygen gas required will be: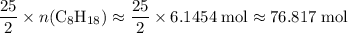 .
.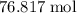 of
of 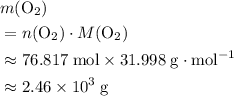 .
.

