
Chemistry, 19.05.2020 02:01 mathbrain58
For the following reaction, if you have 13.2 g of CO and 42.7g of Fe2O3, which is the limiting reagent with regards to Fe production?
Fe2O3 (s) + 3 CO (g) > 2 Fe (s) + 3 CO2 (g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
For the following reaction, if you have 13.2 g of CO and 42.7g of Fe2O3, which is the limiting reage...
Questions







Mathematics, 15.01.2021 19:30

Mathematics, 15.01.2021 19:30

Spanish, 15.01.2021 19:30

Chemistry, 15.01.2021 19:30

Mathematics, 15.01.2021 19:30


Mathematics, 15.01.2021 19:30

Mathematics, 15.01.2021 19:30


English, 15.01.2021 19:30

Mathematics, 15.01.2021 19:30

Social Studies, 15.01.2021 19:30


Mathematics, 15.01.2021 19:30

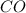 is the limiting reagent and
is the limiting reagent and 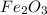 is the excess reagent.
is the excess reagent.
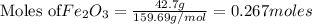
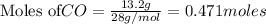
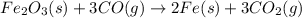
 of
of 

