A weak acid has a Ka equal to 3.2 x 10^-6 what is the pH of a 1M solution of this acid?
...

Chemistry, 16.05.2020 11:57 khalidalrasheedi2025
A weak acid has a Ka equal to 3.2 x 10^-6 what is the pH of a 1M solution of this acid?
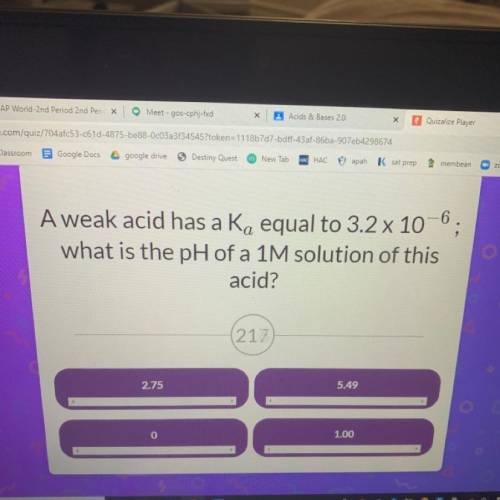

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 23.06.2019 13:30
What would happen if no were added to n(g)+o2=2no(g) at equilibrium?
Answers: 1

Chemistry, 23.06.2019 13:30
The two isotopes of chlorine are 3517cl and 3717cl. which isotope is the most abundant?
Answers: 1
You know the right answer?
Questions

Mathematics, 25.05.2021 08:30


Mathematics, 25.05.2021 08:30

Mathematics, 25.05.2021 08:30


English, 25.05.2021 08:30



Mathematics, 25.05.2021 08:30

Health, 25.05.2021 08:30


Chemistry, 25.05.2021 08:30

Mathematics, 25.05.2021 08:30


English, 25.05.2021 08:30




Geography, 25.05.2021 08:30



