
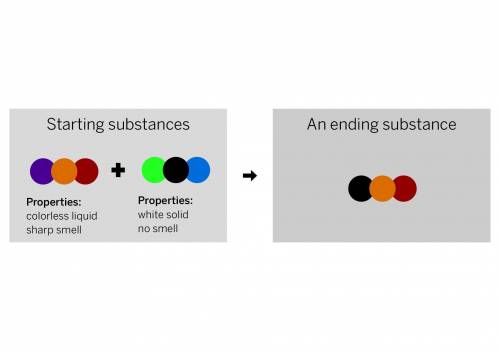
A chemist mixed two substances together: a colorless liquid with a strong smell and a white solid with no smell. The substances’ repeating groups of atoms are shown above on the left. After they were mixed, the chemist analyzed the results and found two substances. One ending substance had the repeating group of atoms shown above on the right. Is the ending substance the same substance as the colorless liquid? What happened to the atoms of the starting substances when the ending substances formed? Be sure to explain your answers to both of these questions.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2


Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
A chemist mixed two substances together: a colorless liquid with a strong smell and a white solid wi...
Questions

Spanish, 05.05.2020 03:40



History, 05.05.2020 03:41

History, 05.05.2020 03:41

Mathematics, 05.05.2020 03:41

Mathematics, 05.05.2020 03:41



History, 05.05.2020 03:41

History, 05.05.2020 03:41







Mathematics, 05.05.2020 03:41

Physics, 05.05.2020 03:41

Mathematics, 05.05.2020 03:41



