A ball has a volume of 6.35 liters and is at a temperature of
27.0°C. A pressure gauge attache...

Chemistry, 10.05.2020 06:57 ShugarLove4363
A ball has a volume of 6.35 liters and is at a temperature of
27.0°C. A pressure gauge attached to the ball reads 0.45
atmosphere. The atmospheric pressure is 1.00 atmosphere.
Calculate the absolute pressure inside the ball and the
amount of moles of air it contains. (First, find the absolute
pressure and then use that to find the moles using the ideal
gas law. Remember to use the correct ideal gas constant and
to convert from celsius to kelvin.)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
Questions



Mathematics, 28.09.2019 21:30






Mathematics, 28.09.2019 21:30







Biology, 28.09.2019 21:30


English, 28.09.2019 21:30

Mathematics, 28.09.2019 21:30

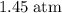 .Number of moles of air particles inside the ball, by the ideal gas law: approximately
.Number of moles of air particles inside the ball, by the ideal gas law: approximately  .
. 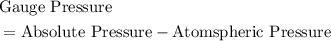 .
.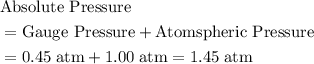 .
. for pressure and
for pressure and  for volume.
for volume.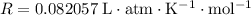 .
. .
.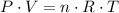 (after rearranging) to find the number of moles of gas particles in this ball:
(after rearranging) to find the number of moles of gas particles in this ball: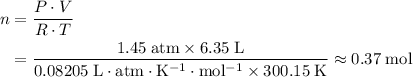 .
.

