
Which of the following best describes what causes water molecules to exhibit properties such as cohesive and adhesive behavior?
a. water molecules are polar, having partially positive and partially negative ends.
b. water molecules have both a hydrophobic end and a hydrophilic end.
c. water molecules are capable of dissolving both polar and nonpolar substances.
d. water molecules have a high molecular weight, making them very dense.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
Which of the following best describes what causes water molecules to exhibit properties such as cohe...
Questions

Mathematics, 30.04.2021 17:20


Physics, 30.04.2021 17:20

Mathematics, 30.04.2021 17:20


History, 30.04.2021 17:20


Biology, 30.04.2021 17:20

Mathematics, 30.04.2021 17:20


Mathematics, 30.04.2021 17:20



Mathematics, 30.04.2021 17:20


English, 30.04.2021 17:20

Chemistry, 30.04.2021 17:20



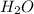 . It is a polar covalent molecule and in each O-H bond, oxygen has a partial negative charge and hydrogen a partial positive charge. Water molecules have strong cohesive forces as they can form intermolecular hydrogen bonds. Water molecules can also show strong adhesive forces with the walls of the container it holds due to the polar nature of the molecules.
. It is a polar covalent molecule and in each O-H bond, oxygen has a partial negative charge and hydrogen a partial positive charge. Water molecules have strong cohesive forces as they can form intermolecular hydrogen bonds. Water molecules can also show strong adhesive forces with the walls of the container it holds due to the polar nature of the molecules. 

