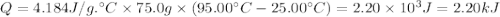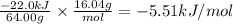Chemistry, 07.05.2020 12:58 dinadenoirefan
What is the MOLAR heat of combustion of methane(CH₄) if 64.00g of methane are burned to heat 75.0 ml of water from 25.00°C to 95.00°C?(Water heat capacity-4.184 J/g°C)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
What is the MOLAR heat of combustion of methane(CH₄) if 64.00g of methane are burned to heat 75.0 ml...
Questions

History, 23.04.2020 01:51


Mathematics, 23.04.2020 01:51





Mathematics, 23.04.2020 01:51

Biology, 23.04.2020 01:51


Mathematics, 23.04.2020 01:51


English, 23.04.2020 01:51



Mathematics, 23.04.2020 01:51


Computers and Technology, 23.04.2020 01:51