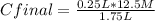Chemistry, 07.05.2020 08:57 NathanaelLopez
If I add 1.50 L of water to 250 mL of a 12.5 M NaOH solution, what will the molarity of the diluted solution be?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

You know the right answer?
If I add 1.50 L of water to 250 mL of a 12.5 M NaOH solution, what will the molarity of the diluted...
Questions



Chemistry, 09.03.2021 03:00

Mathematics, 09.03.2021 03:00


Mathematics, 09.03.2021 03:00

English, 09.03.2021 03:00


Mathematics, 09.03.2021 03:00


English, 09.03.2021 03:00


Mathematics, 09.03.2021 03:00





Mathematics, 09.03.2021 03:00

Social Studies, 09.03.2021 03:00