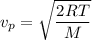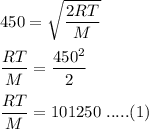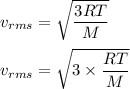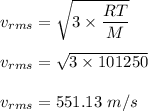Chemistry, 07.05.2020 04:58 justijust500
The most probable speed of an oxygen molecule in the gas phase at room temperature is 450 m/s. The root-mean-square speed (urms) is therefore equal to 450 m/s. much greater than 450 m/s. slightly less than 450 m/s. much less than 450 m/s. slightly greater than 450 m/s.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
The most probable speed of an oxygen molecule in the gas phase at room temperature is 450 m/s. The r...
Questions


Mathematics, 12.11.2019 20:31

Mathematics, 12.11.2019 20:31

Mathematics, 12.11.2019 20:31

History, 12.11.2019 20:31

Mathematics, 12.11.2019 20:31

Health, 12.11.2019 20:31

Biology, 12.11.2019 20:31


Computers and Technology, 12.11.2019 20:31





Mathematics, 12.11.2019 20:31


Mathematics, 12.11.2019 20:31

Biology, 12.11.2019 20:31

History, 12.11.2019 20:31

Mathematics, 12.11.2019 20:31