Chemistry, 07.05.2020 05:00 tordiacasey
Consider an electrolytic cell with a platinum anode and a silver cathode in a 1.0 M AgNO3(aq) solution.
a) (3 pts) What species can be reduced in this solution? Which species is preferentially reduced? Write the reduction half- reaction. (Note that oxyanions like nitrate are not commonly reduced in aqueous electrolysis due to kinetic reasons.)
b) (2 pts) Which species is oxidized during the electrolysis? Write the oxidation half-reaction. Note that acid will form as a byproduct of the oxidation.
c) (2 pts) Write the overall electrolysis reaction in net ionic and molecular forms.
d) (2 pts) Determine the external electric potential needed for the electrolysis under standard conditions.
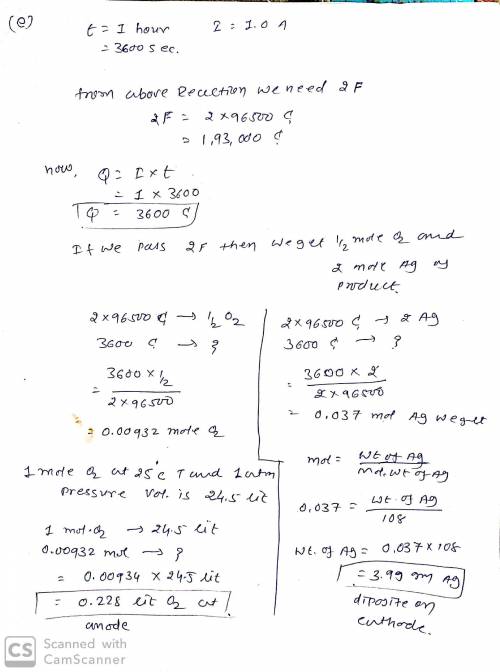
e) (5 pts) If the electrolysis is carried out for 1.00 hour using 1.00 A current, how many grams of metal will be deposited at the cathode and how many liters of gas will form at the anode at 1.00 atm pressure and 25°C?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

You know the right answer?
Consider an electrolytic cell with a platinum anode and a silver cathode in a 1.0 M AgNO3(aq) soluti...
Questions





Social Studies, 03.03.2020 01:22

Mathematics, 03.03.2020 01:22



Mathematics, 03.03.2020 01:22





Physics, 03.03.2020 01:22

Mathematics, 03.03.2020 01:22