Chemistry, 07.05.2020 03:11 parrazm2022
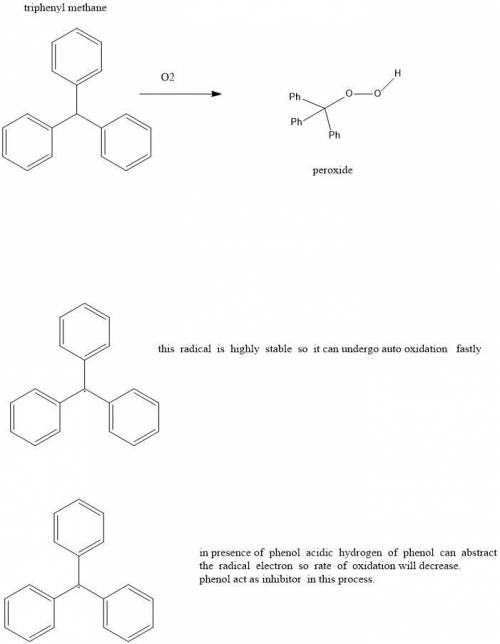
10.31 Triphenylmethane readily undergoes autooxidation to produce a hydroperoxide: c10s172 (a) Draw the expected hydroperoxide. (b) Explain why triphenylmethane is so susceptible to autooxidation. (c) In the presence of phenol (C6H5OH), triphenylmethane undergoes autooxidation at a much slower rate. Explain this observation.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
10.31 Triphenylmethane readily undergoes autooxidation to produce a hydroperoxide: c10s172 (a) Draw...
Questions




Chemistry, 02.10.2020 20:01

English, 02.10.2020 20:01



Biology, 02.10.2020 20:01

Mathematics, 02.10.2020 20:01


Business, 02.10.2020 20:01

History, 02.10.2020 20:01






Mathematics, 02.10.2020 20:01

Mathematics, 02.10.2020 20:01

Mathematics, 02.10.2020 20:01