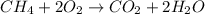Chemistry, 06.05.2020 23:07 Trucofer2106
Acetylene gas (C2H2) reacts with oxygen to produce carbon dioxide and water. When 30.0 g of acetylene is reacted with O2, 18.5 g of water is formed.
2 C2H2 (g) + 3 O2 (g) → 2 CO2 (g) + 2 H2O (l)
What type of reaction is this?
What is the percent yield of this reaction?

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1

Chemistry, 23.06.2019 10:30
Chemical bonds result from the interaction of the from two or more atoms. a. protons b. electrons c. neutrons d. nuclei
Answers: 2

Chemistry, 23.06.2019 15:00
What is the volume in liters of 7500 g of helium atoms. assume stp conditions.
Answers: 1
You know the right answer?
Acetylene gas (C2H2) reacts with oxygen to produce carbon dioxide and water. When 30.0 g of acetylen...
Questions

Mathematics, 12.02.2021 20:30

Spanish, 12.02.2021 20:30





Mathematics, 12.02.2021 20:30



Mathematics, 12.02.2021 20:30

Arts, 12.02.2021 20:30


Mathematics, 12.02.2021 20:30




Mathematics, 12.02.2021 20:30



Mathematics, 12.02.2021 20:30