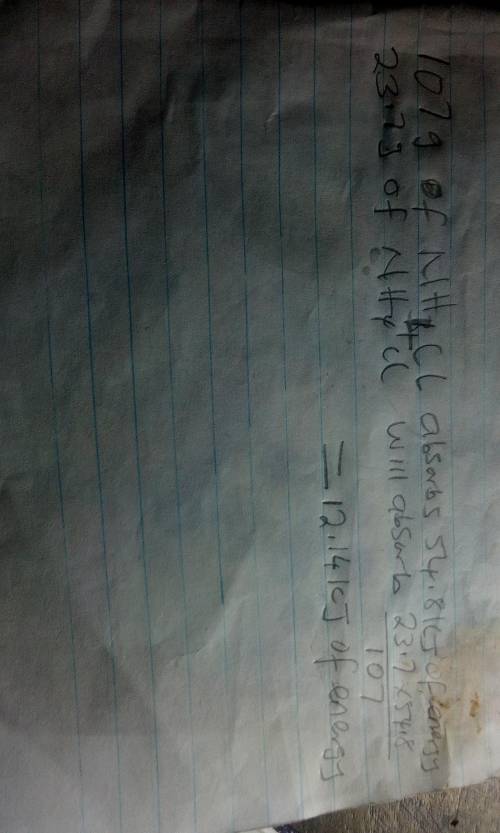Chemistry, 03.02.2020 20:45 PrincessIndia
The energy diagram shown represents the chemical reaction between solid ammonium chloride and solid barium hydroxide octahydrate:
2nh4cl(s) + ba(oh)2 x 8h2o(> 2nh3(aq)+bacl2(aq)+10h2o(1)
the (triangle then an h) for this reaction is 54.8 kj. how much energy would be absorbed if 23.7 g of nh4cl reacts?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
The energy diagram shown represents the chemical reaction between solid ammonium chloride and solid...
Questions

Chemistry, 08.12.2020 21:50




Mathematics, 08.12.2020 21:50


Mathematics, 08.12.2020 21:50

World Languages, 08.12.2020 21:50

Mathematics, 08.12.2020 21:50


Mathematics, 08.12.2020 21:50

Mathematics, 08.12.2020 21:50



Arts, 08.12.2020 21:50

History, 08.12.2020 21:50


Mathematics, 08.12.2020 21:50


English, 08.12.2020 21:50