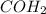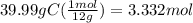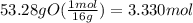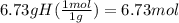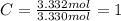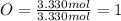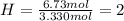Chemistry, 06.05.2020 07:45 jesh0975556
Determine the empirical formula of a compound that is 39.99% carbon, 53.28% Oxygen and 6.73% Hydrogen

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1

You know the right answer?
Determine the empirical formula of a compound that is 39.99% carbon, 53.28% Oxygen and 6.73% Hydroge...
Questions







Biology, 14.12.2020 03:40


History, 14.12.2020 03:40




Social Studies, 14.12.2020 03:40

Mathematics, 14.12.2020 03:50

Mathematics, 14.12.2020 03:50


Mathematics, 14.12.2020 03:50


History, 14.12.2020 03:50