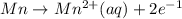A galvanic cell consists of one half-cell that contains Ag(s) and Ag+(aq), and one half-cell that contains Mn(s) and Mn2+(aq). What species are produced at the electrodes under standard conditions? Ag+(aq) + e- → Ag(s) E° = +0.80 V Mn2+(aq) + 2 e- → Mn(s) E° = -1.18 V A) Ag(aq) is formed at the cathode and, Mn(s) is formed at the anode. B) Ag(s) is formed at the cathode, and Mn2+(aq) is formed at the anode. C) Mn(s) is formed at the cathode, and Ag+(aq) is formed at the anode. D) Mn2+(aq) is formed at the cathode, and Ag(s) is formed at the anode.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
A galvanic cell consists of one half-cell that contains Ag(s) and Ag+(aq), and one half-cell that co...
Questions


Mathematics, 20.03.2021 22:00

Mathematics, 20.03.2021 22:00


Arts, 20.03.2021 22:00

Mathematics, 20.03.2021 22:00

Social Studies, 20.03.2021 22:00





Mathematics, 20.03.2021 22:00


Mathematics, 20.03.2021 22:00


History, 20.03.2021 22:00




Business, 20.03.2021 22:00

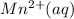 is formed at the anode.
is formed at the anode. 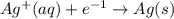 E=0.80 V
E=0.80 V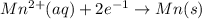 E=-1.18 V
E=-1.18 V