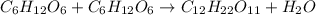Consider the following reaction.
c6h12o6+c6h12o6 -- c12h22o11
a. the equat...

Chemistry, 12.01.2020 01:31 mikayleighb2019
Consider the following reaction.
c6h12o6+c6h12o6 -- c12h22o11
a. the equation is not balanced; it is missing a molecule of water. write it in on the correct side of the equation.
b. what kind of reaction is this?
c. is c6h12o6 (glucose) a monomer, or a polymer?
d. to summarize, when two monomers are joined, a molecule of is always removed.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
Questions


Computers and Technology, 01.10.2021 05:40


Mathematics, 01.10.2021 05:40

Mathematics, 01.10.2021 05:40

Mathematics, 01.10.2021 05:40

Mathematics, 01.10.2021 05:40

Social Studies, 01.10.2021 05:40

Social Studies, 01.10.2021 05:40

Mathematics, 01.10.2021 05:40


Mathematics, 01.10.2021 05:40


Law, 01.10.2021 05:40

Social Studies, 01.10.2021 05:40


Health, 01.10.2021 05:40

Chemistry, 01.10.2021 05:40