
Match the following aqueous solutions with the appropriate letter from the column on the right. Assume complete dissociation of electrolytes. 1. 0.17 m NH4CH3COO A. Lowest freezing point 2. 0.18 m MnSO4 B. Second lowest freezing point 3. 0.20 m CoSO4 C. Third lowest freezing point 4. 0.42 m Ethylene glycol (nonelectrolyte) D. Highest freezing point

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
Match the following aqueous solutions with the appropriate letter from the column on the right. Assu...
Questions





Computers and Technology, 20.12.2019 19:31

History, 20.12.2019 19:31

Health, 20.12.2019 19:31

Chemistry, 20.12.2019 19:31


Business, 20.12.2019 19:31


History, 20.12.2019 19:31

History, 20.12.2019 19:31

Mathematics, 20.12.2019 19:31


Chemistry, 20.12.2019 19:31



Social Studies, 20.12.2019 19:31

Physics, 20.12.2019 19:31

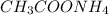 : Highest freezing point
: Highest freezing point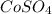 : Second lowest freezing point
: Second lowest freezing point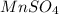 : Third lowest freezing point
: Third lowest freezing point 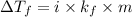
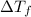 = change in freezing point
= change in freezing point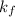 = freezing point constant
= freezing point constant

