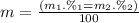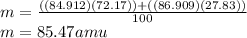Chemistry, 27.08.2019 07:00 Jerrikasmith28
Given that rubidium has two isotopes, 85rb and 87rb. calculate the average atomic mass of rubidium.
note that 85rb has an atomic mass of 84.912 amu and occurs at an abundance of 72.17% while 87rb has an atomic mass of 86.909 amu and occurs at an abundance of 27.83%.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1


Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Given that rubidium has two isotopes, 85rb and 87rb. calculate the average atomic mass of rubidium.<...
Questions

Mathematics, 30.05.2021 07:20

English, 30.05.2021 07:20





Social Studies, 30.05.2021 07:20

Mathematics, 30.05.2021 07:20

Mathematics, 30.05.2021 07:20


Mathematics, 30.05.2021 07:20


English, 30.05.2021 07:20

Mathematics, 30.05.2021 07:20

Mathematics, 30.05.2021 07:20


Chemistry, 30.05.2021 07:20

History, 30.05.2021 07:30

Computers and Technology, 30.05.2021 07:30

Mathematics, 30.05.2021 07:30