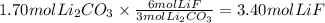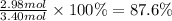The chemical reaction between lithium carbonate and aluminum trifluoride produces aluminum carbonate and lithium fluoride:
2AlF3 + 3Li2CO3 → Al2(CO3)3 + 6LiF.
You have an excess of aluminum trifluoride and 1.70 moles of lithium carbonate, which produces 2.98 moles of lithium fluoride. What is the percent yield of the reaction? Use the periodic table and this polyatomic ion resource.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
The chemical reaction between lithium carbonate and aluminum trifluoride produces aluminum carbonate...
Questions

History, 10.04.2020 21:34

Mathematics, 10.04.2020 21:34










Chemistry, 10.04.2020 21:35


Computers and Technology, 10.04.2020 21:35


Mathematics, 10.04.2020 21:35

History, 10.04.2020 21:35



Computers and Technology, 10.04.2020 21:35