
Under certain conditions the rate of this reaction is zero order in ammonia with a rate constant of ·0.0038Ms−1: 2NH3(g)→N2(g)+3H2(g) Suppose a 450.mL flask is charged under these conditions with 150.mmol of ammonia. How much is left 20.s later? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
Under certain conditions the rate of this reaction is zero order in ammonia with a rate constant of...
Questions


Mathematics, 14.05.2021 16:20

Mathematics, 14.05.2021 16:20

Physics, 14.05.2021 16:20


Chemistry, 14.05.2021 16:20

Mathematics, 14.05.2021 16:20

Geography, 14.05.2021 16:20


Computers and Technology, 14.05.2021 16:20



Social Studies, 14.05.2021 16:30

Mathematics, 14.05.2021 16:30


Social Studies, 14.05.2021 16:30

English, 14.05.2021 16:30

Mathematics, 14.05.2021 16:30

History, 14.05.2021 16:30

Mathematics, 14.05.2021 16:30

 mmol of
mmol of 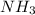 is left after 20 s.
is left after 20 s.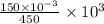 M = 0.333 M
M = 0.333 M![[NH_{3}]=-kt+[NH_{3}]_{0}](/tpl/images/0641/7445/9cd63.png)
![[NH_{3}]](/tpl/images/0641/7445/acd38.png) represents concentration of
represents concentration of ![[NH_{3}]_{0}](/tpl/images/0641/7445/b342c.png) is initial concentration of
is initial concentration of ![[NH_{3}]=(-0.0038M.s^{-1}\times 20s)+0.333M](/tpl/images/0641/7445/bbef3.png)
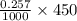 mol = 0.11565 mol
mol = 0.11565 mol

