
Chemistry, 05.05.2020 18:40 lillianneal
Substance ΔG°f(kJ/mol) M3O4(s) −8.80 M(s) 0 O2(g) 0 Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M3O4(s)↽−−⇀ 3M(s)+2O2(g) What is the standard change in Gibbs energy for the reaction, as written, in the forward direction? ΔG∘rxn= kJ/mol What is the equilibrium constant of this reaction, as written, in the forward direction at 298 K? K= What is the equilibrium pressure of O2(g) over M(s) at 298 K?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

You know the right answer?
Substance ΔG°f(kJ/mol) M3O4(s) −8.80 M(s) 0 O2(g) 0 Consider the decomposition of a metal oxide to i...
Questions


Mathematics, 16.12.2020 20:40

Advanced Placement (AP), 16.12.2020 20:40

Physics, 16.12.2020 20:40







Mathematics, 16.12.2020 20:40

Mathematics, 16.12.2020 20:40

Biology, 16.12.2020 20:40



English, 16.12.2020 20:40

Social Studies, 16.12.2020 20:40

Mathematics, 16.12.2020 20:40

Mathematics, 16.12.2020 20:40

Mathematics, 16.12.2020 20:40

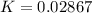
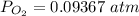
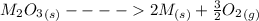
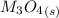 is
is 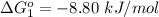
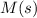 is
is 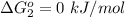
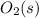 is
is 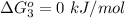
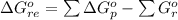
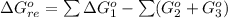
![\Delta G^o_{re} =[ (2 * 0) + (\frac{3}{2} * 0 )] - [1 * - 8.80]](/tpl/images/0641/3844/374c7.png)
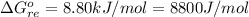

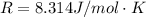
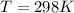
![K_p =[ P_{O_2}]^{\frac{3}{2} }](/tpl/images/0641/3844/66043.png)
![[ P_{O_2}]](/tpl/images/0641/3844/bc839.png) is the equilibrium pressure of oxygen
is the equilibrium pressure of oxygen 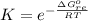
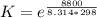
![0.02867 = [P_{O_2}]^{[\frac{3}{2} ]}](/tpl/images/0641/3844/d1a4b.png)
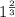
![P_{O_2} = [0.02867]^{\frac{2}{3} }](/tpl/images/0641/3844/dba69.png)


