
Chemistry, 05.05.2020 17:44 gapaxton22
The heat capacity of an object is given by the following equation: C equals 14000 straight J over straight K plus open parentheses 200 straight J over straight K squared close parentheses T plus open parentheses 3 straight J over straight K cubed close parentheses T squared What is the change in the entropy of the object (in J/K) associated with raising its temperature from 290 K to 380 K?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1

Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 2
You know the right answer?
The heat capacity of an object is given by the following equation: C equals 14000 straight J over st...
Questions

Geography, 02.12.2020 23:00

Social Studies, 02.12.2020 23:00


Mathematics, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00




Mathematics, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00

History, 02.12.2020 23:00


History, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00





![C = 14000\frac{J}{K} + (200 \frac{J}{K^2} )T + [3 \frac{J}{K^3} ] T^2](/tpl/images/0640/9264/78425.png)
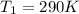
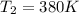
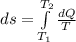
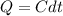


![ds = 1400\ ln [T]+ 200 \frac{T^2}{T} +3 \frac{T^2}{2 T} \ \ | \left \ T_2} \atop {T_1}} \right.](/tpl/images/0640/9264/c257a.png)
![ds = 1400 ln [\frac{T_2}{T_1} ] + 200 (T_2 - T_1 ) + \frac{3}{2} (T_2^2 -T_1^2)](/tpl/images/0640/9264/6b21c.png)
![ds = 1400 ln [\frac{380}{290} ] + 200 (380 - 290 ) + \frac{3}{2} (380^2 -290 ^2)](/tpl/images/0640/9264/2ea63.png)


