Chemistry, 05.05.2020 17:41 tyresharichardson29
The ratio of carbon-14 to carbon-12 in the shaft of a wooden arrow, unearthed when a foundation was being dug for a new house, is 56.0% of the same ratio in a growing tree today. Assuming the ratio of carbon-14 to carbon-12 in the atmosphere has been constant, calculate the age of the arrow. The half-life of carbon-14 is 5730 years. The arrow is years old Numeric

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1
You know the right answer?
The ratio of carbon-14 to carbon-12 in the shaft of a wooden arrow, unearthed when a foundation was...
Questions


English, 29.10.2019 20:31





Mathematics, 29.10.2019 20:31


Chemistry, 29.10.2019 20:31




Social Studies, 29.10.2019 20:31

Physics, 29.10.2019 20:31



Biology, 29.10.2019 20:31

History, 29.10.2019 20:31


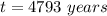 old
old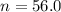 %
%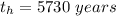
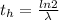
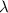 is the decay constant
is the decay constant 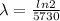
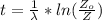
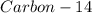 is
is 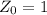
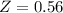 %
%