Chemistry, 05.05.2020 17:37 canyonmorlan
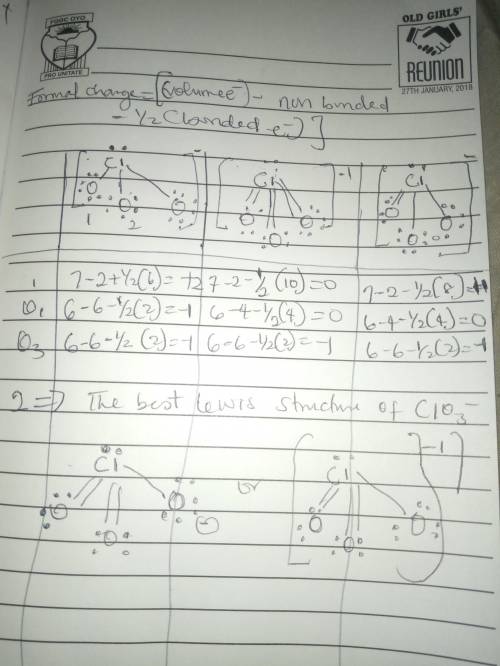
The formal charge is the "charge" an element would have in a molecule or ion if all of the bonding electrons were shared equally between atoms. We can draw three inequivalent Lewis structures for the thiocyanate ion , SCN- . The concepts of formal charge and electronegativity can help us choose the structure that is the best representation.1. Assign formal charges to the elements in each of the structures below. ABCFormal ChargeSCN-12010-100-12. The best Lewis structure for SCN- is .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 23.06.2019 05:00
Scientists discovered fossils in several layers of the earth you see here. they found fossils of algae, snails, and clams in layer d. given that information, where do you think they found fossil evidence of simple land plants and amphibians?
Answers: 1

Chemistry, 23.06.2019 13:10
When can a hypothesis be elevated to the status of a theory? a) when it is validated by an experiment b) when data gathered from an experiment precisely fits predictions c) when it can be proved to be true d) when it meets the test of repeated experimentation
Answers: 2
You know the right answer?
The formal charge is the "charge" an element would have in a molecule or ion if all of the bonding e...
Questions

Mathematics, 12.02.2020 07:53

Mathematics, 12.02.2020 07:53

Mathematics, 12.02.2020 07:54

Mathematics, 12.02.2020 07:54


Mathematics, 12.02.2020 07:54


Mathematics, 12.02.2020 07:54

Mathematics, 12.02.2020 07:54


Biology, 12.02.2020 07:54

Social Studies, 12.02.2020 07:54


English, 12.02.2020 07:55

Mathematics, 12.02.2020 07:55

Mathematics, 12.02.2020 07:55

Mathematics, 12.02.2020 07:55

Mathematics, 12.02.2020 07:56

Mathematics, 12.02.2020 07:56

Mathematics, 12.02.2020 07:57