
Chemistry, 05.05.2020 09:46 kezionhoward13
Identify the correct set-up. How many moles of aluminum oxide would form if 25.0 g of aluminum react completely given the following chemical reaction: 4Al + 3O₂ → 2Al₂O₃?
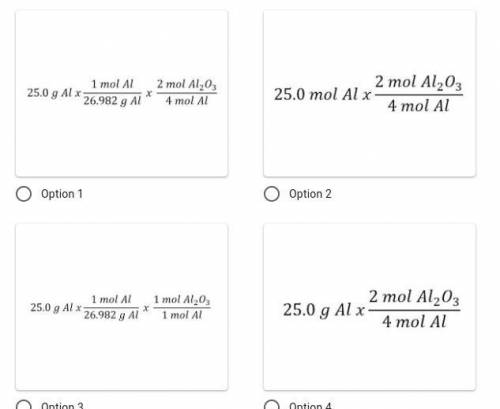

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
Identify the correct set-up. How many moles of aluminum oxide would form if 25.0 g of aluminum react...
Questions

Geography, 13.11.2020 22:50

History, 13.11.2020 22:50




Mathematics, 13.11.2020 22:50




Biology, 13.11.2020 22:50

Computers and Technology, 13.11.2020 22:50

History, 13.11.2020 22:50


Computers and Technology, 13.11.2020 22:50

Mathematics, 13.11.2020 22:50

Mathematics, 13.11.2020 22:50

English, 13.11.2020 22:50

Business, 13.11.2020 22:50


Mathematics, 13.11.2020 22:50



