Chemistry, 05.05.2020 00:29 stefaniethibodeaux
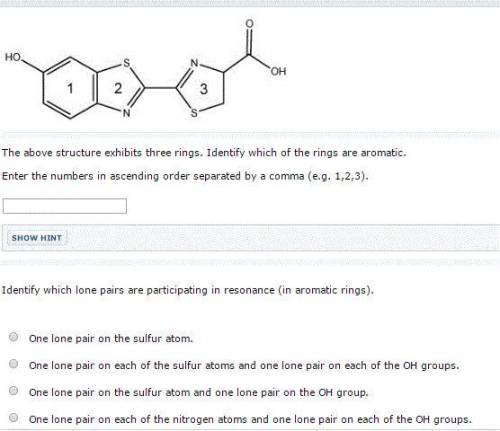
The above structure exhibits three rings. Identify which of the rings are aromatic. Enter the numbers in ascending order separated by a comma (e. g. 1,2,3). Identify which lone pairs are participating in resonance (in aromatic rings). One lone pair on each of the nitrogen atoms and one lone pair on each of the OH groups. One lone pair on the sulfur atom. One lone pair on the sulfur atom and one lone pair on the OH group. One lone pair on each of the sulfur atoms and one lone pair on each of the OH groups

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

You know the right answer?
The above structure exhibits three rings. Identify which of the rings are aromatic. Enter the number...
Questions

Social Studies, 07.03.2020 05:38









Computers and Technology, 07.03.2020 05:38



History, 07.03.2020 05:38



Computers and Technology, 07.03.2020 05:38