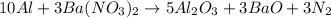Chemistry, 03.02.2020 14:00 trying2passs
In some fireworks there is a reaction between powdered aluminium and powdered barium nitrate in which heat is evolved and an unreactive gas is produced.
what is the equation for this reaction?
a. 2al + ba(no3)2 -> al2o3 + bao + 2no
b. 4al + 4ba(no3)2 -> 2al2o3 + 4 ba(no2)2 + o2
c. 10al + 3 ba(no3)2 -> 5al2o3 + 3bao + 3n2
d. 10al + 18ba(no3)2 -> 10al(no3)3 +18bao +3n2

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
In some fireworks there is a reaction between powdered aluminium and powdered barium nitrate in which...
Questions


Biology, 19.07.2019 10:10


Biology, 19.07.2019 10:10



Computers and Technology, 19.07.2019 10:10

Spanish, 19.07.2019 10:10



Mathematics, 19.07.2019 10:10

English, 19.07.2019 10:10

History, 19.07.2019 10:10

Physics, 19.07.2019 10:10





History, 19.07.2019 10:10