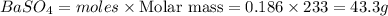Chemistry, 13.01.2020 23:31 bullsfan4584
Calculate the mass of the precipitate formed when 2.27 l of 0.0820 m ba(oh)2 are mixed with 3.06 l of 0.0664 m na2so4.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
Calculate the mass of the precipitate formed when 2.27 l of 0.0820 m ba(oh)2 are mixed with 3.06 l o...
Questions

Physics, 18.05.2021 14:00

Biology, 18.05.2021 14:00


Chemistry, 18.05.2021 14:00


Computers and Technology, 18.05.2021 14:00

Physics, 18.05.2021 14:00

Mathematics, 18.05.2021 14:00

Spanish, 18.05.2021 14:00

Mathematics, 18.05.2021 14:00



Mathematics, 18.05.2021 14:00



Social Studies, 18.05.2021 14:00



Computers and Technology, 18.05.2021 14:00

Business, 18.05.2021 14:00

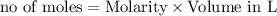


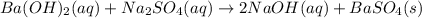
 reacts with 1 mole of
reacts with 1 mole of 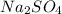
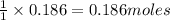 of
of 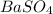 precipitate
precipitate