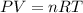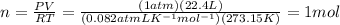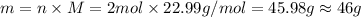Chemistry, 09.10.2019 09:30 nellyjsotelo
According to the equation 2na + 2h2o mc012-1.jpg 2naoh+h2, what mass of na is required to yield 22.4 l of h2 at stp? (the atomic mass of na is 22.99 a. 1.00 g. b. 2.00 g. c. 23.0 g. d. 46.0 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 04:40
Listen base your answer to the question on the information below.propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below.c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol.what is the correct structural formula for a molecule of methanethiol
Answers: 3

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3
You know the right answer?
According to the equation 2na + 2h2o mc012-1.jpg 2naoh+h2, what mass of na is required to yield 22.4...
Questions



Chemistry, 19.12.2019 14:31

Spanish, 19.12.2019 14:31


Computers and Technology, 19.12.2019 14:31


Mathematics, 19.12.2019 14:31

Health, 19.12.2019 14:31


Chemistry, 19.12.2019 15:31

Chemistry, 19.12.2019 15:31


History, 19.12.2019 15:31


Mathematics, 19.12.2019 15:31

History, 19.12.2019 15:31



History, 19.12.2019 15:31

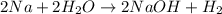
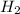 formed at STP,
formed at STP,