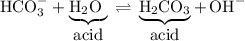Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
You know the right answer?
Which compounds are acting like Brønsted-Lowry acids in the following acid-base equilibrium?
Questions

Mathematics, 10.12.2020 21:10

Mathematics, 10.12.2020 21:10


Mathematics, 10.12.2020 21:10




Mathematics, 10.12.2020 21:10

Mathematics, 10.12.2020 21:10



Mathematics, 10.12.2020 21:10

Mathematics, 10.12.2020 21:10

History, 10.12.2020 21:10

History, 10.12.2020 21:10


Chemistry, 10.12.2020 21:10

English, 10.12.2020 21:10


History, 10.12.2020 21:10