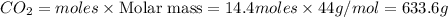Chemistry, 03.05.2020 14:16 jaqwannewsome
What mass of CO2 can be produced from the decomposition of 4.8 moles of Fe2(CO3)3?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
What mass of CO2 can be produced from the decomposition of 4.8 moles of Fe2(CO3)3?...
Questions

History, 29.08.2019 01:40



History, 29.08.2019 01:40


Mathematics, 29.08.2019 01:40

Computers and Technology, 29.08.2019 01:40


Mathematics, 29.08.2019 01:40



Mathematics, 29.08.2019 01:40


Mathematics, 29.08.2019 01:40



History, 29.08.2019 01:40

History, 29.08.2019 01:40


Social Studies, 29.08.2019 01:50

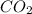 can be produced from the decomposition of 4.8 moles of
can be produced from the decomposition of 4.8 moles of 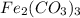
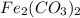 is :
is :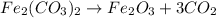
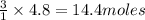 of
of