
Chemistry, 03.05.2020 13:04 Kaziyah461
0.80 mol MgBr2 is added to 1.00 kg water. Determine the freezing point of the solution. Water has a
freezing point depression constant of 1.86°C. kg/mol.
Which equation should you use?
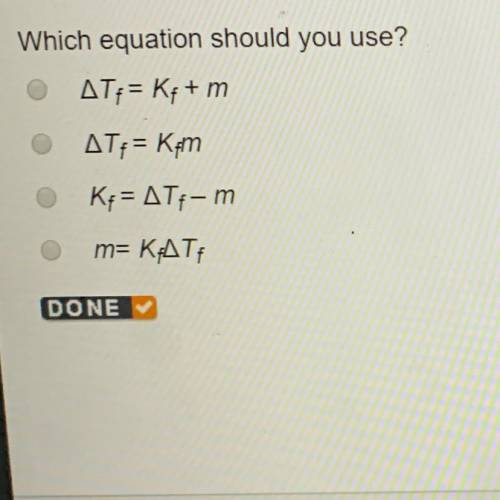

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
0.80 mol MgBr2 is added to 1.00 kg water. Determine the freezing point of the solution. Water has a<...
Questions


Mathematics, 08.11.2020 06:20





Arts, 08.11.2020 06:20

Mathematics, 08.11.2020 06:20



English, 08.11.2020 06:20


Mathematics, 08.11.2020 06:20


Medicine, 08.11.2020 06:20

Mathematics, 08.11.2020 06:20




Arts, 08.11.2020 06:20



