Calculate the hydronium ion concentration in an aqueous solution with a pH of 11.7 at
25°C....


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
Questions






Spanish, 16.10.2020 17:01



Mathematics, 16.10.2020 17:01

Business, 16.10.2020 17:01



Mathematics, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01





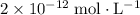 .
.![\left[\mathrm{H_3O^{+}}\right]](/tpl/images/0629/7930/17c4d.png) of an aqueous solution can be found from its
of an aqueous solution can be found from its 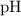 with the equation:
with the equation:![\displaystyle \left[\mathrm{H_3O^{+}}\right] = 10^{-\mathrm{pH}}](/tpl/images/0629/7930/3c39f.png) .
.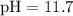 . Hence,
. Hence,![\begin{aligned}& \left[\mathrm{H_3O^{+}}\right] \\ &= 10^{-\mathrm{pH}} \\ &= 10^{-11.7} \approx 2 \times 10^{-12}\end{aligned}](/tpl/images/0629/7930/10c0e.png) .
. 

