Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3


Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1
You know the right answer?
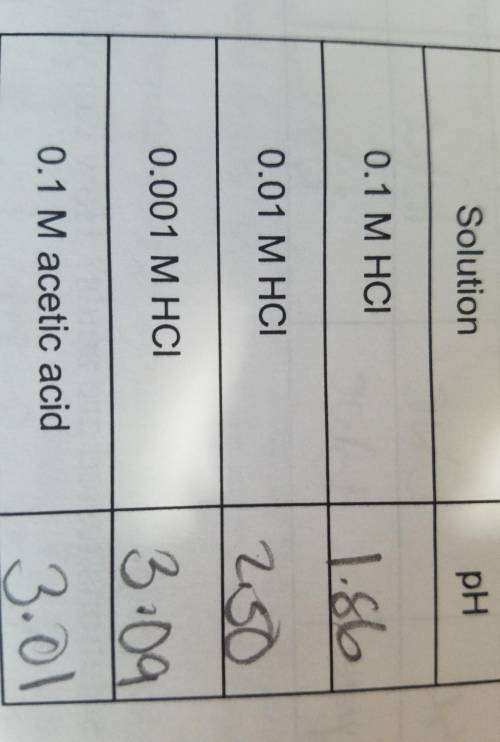
Explain why the 0.1 M HC1 and acetic acid solutions have different pH values despite having the dame...
Questions

Chemistry, 21.01.2021 23:50

Mathematics, 21.01.2021 23:50



Mathematics, 21.01.2021 23:50

Mathematics, 21.01.2021 23:50

Mathematics, 21.01.2021 23:50


Mathematics, 21.01.2021 23:50


Social Studies, 21.01.2021 23:50



History, 21.01.2021 23:50


Mathematics, 21.01.2021 23:50


Mathematics, 22.01.2021 01:00

Health, 22.01.2021 01:00

Biology, 22.01.2021 01:00