HELP ASAP PLZ
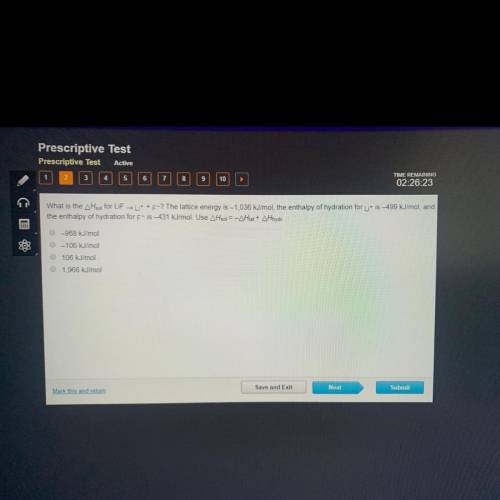
What is the AHsol for LiF it + F-? The lattice energy is -1.036 kJ/mol, t...

HELP ASAP PLZ
What is the AHsol for LiF it + F-? The lattice energy is -1.036 kJ/mol, the enthalpy of hydration for it is -499 kJ/mol, and
the enthalpy of hydration for F-is-431 kJ/mol. Use AHsol = -A Hat + AHhydr-
A: -968 kJ/mol
B: -106 kJ/mol
C: 106 kJ/mol
D: 1,966 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

You know the right answer?
Questions



Mathematics, 04.09.2020 22:01

Biology, 04.09.2020 22:01






Mathematics, 04.09.2020 22:01



Physics, 04.09.2020 22:01



Geography, 04.09.2020 22:01

Mathematics, 04.09.2020 22:01


Mathematics, 04.09.2020 22:01

Mathematics, 04.09.2020 22:01



