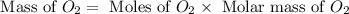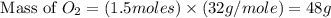Chemistry, 05.05.2020 00:10 emilybomar7466
Given the reaction: 2H₂ + O₂ → 2H₂O The total number of grams of O₂ needed to produce 54 grams of water is .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

You know the right answer?
Given the reaction: 2H₂ + O₂ → 2H₂O The total number of grams of O₂ needed to produce 54 grams of wa...
Questions

Arts, 27.02.2021 01:00




Physics, 27.02.2021 01:00


Mathematics, 27.02.2021 01:00

Mathematics, 27.02.2021 01:00

Mathematics, 27.02.2021 01:00

Spanish, 27.02.2021 01:00

Mathematics, 27.02.2021 01:00


Mathematics, 27.02.2021 01:00

Mathematics, 27.02.2021 01:00


History, 27.02.2021 01:00




History, 27.02.2021 01:00

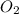 needed are, 48 grams.
needed are, 48 grams.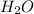
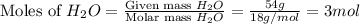
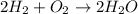
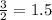 mole of
mole of