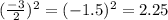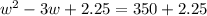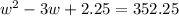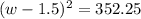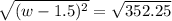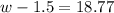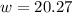Introduction: This activity introduces you to solutions and allows you to experience making different
concentrations of Kool-aid solution. There are many ways to calculate the concentration of a substance
including: molarity (M), parts per million (ppm), percent composition (% comp), and grams per liter (g/L). In
chemistry, concentration is usually measured by the number of moles of substance/ liter of substance, or
Molarity.
Materials:
• Kool-Aid Powder with SUGAR already included (or sugar if you don’t have kool-aid)
• Something to stir solutions
• 4 large cups
• measuring cup (ml preferred but cups will work)
Instructions: In this activity you will be making a series of kool-aid solutions. Make sure to record all data and
answer all the questions below.
Step 1: You will prepare a 237.5 ml kool-aid solution with a concentration of 1M. The molar mass of kool-aid
(or sugar since kool-aid is basically sugar C12H22O11.) is 342 g/mol. Show the calculations below which helped
you create your 1M solution. Note: 237.5 mL is approximately one cup.
Please show your work here.
Liters of water needed
Molarity of kool-aid needed
Grams of kool-aid needed
Now prepare the solution and make sure to label it (sticky note, pen on plastic cup, piece of paper under
cup, etc…)
To make this solution:
1. Measure approximately ½ dry cups of the Kool-aid mixture.
2. Pour the Kool-aid powder you measured into a large glass or cup.
3. Measure out the appropriate amount of water (1 cup) and mix into your glass/cup.
5. Stir to mix and label with the appropriate molarity.
6. Don’t taste test it yet, that will come later.
Do not discard the leftover 1M solution; we are not done with it yet!
Step 2: Using the 1M solution above you will create a second solution with a molarity of 0.5 M solution and
volume of 237.5 mL of kool-aid. Watch video link below for help.
Show your work here. You may need the following equation M1V1=M2V2 where M stands for molarity and V
standards for volume. :
Volume of 1M solution needed
Volume of water needed
Now prepare the solution and make sure to label it! Do not discard the leftover 1M and 0.5 M solutions;
we are not done with them yet!
Step 3: Using the 0.5 M solution above you will create 237.5 mL of a 0.25 M solution of kool-aid. You will need
the following equation M1V1=M2V2 where M stands for molarity and V standards for volume. Note: 237.5 ml is
about 1 cup. There is no video demonstration of this calculation, but follow the same dilution calculation
procedure as was demonstrated in step 2.
Show your work here:
Volume of 0.5 M solution needed:
Volume of water needed:
Now prepare the solution, make sure to label! Do not discard the leftover solutions; we are not done
with them yet!

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2
You know the right answer?
Introduction: This activity introduces you to solutions and allows you to experience making differen...
Questions





Chemistry, 16.06.2020 22:57


Mathematics, 16.06.2020 22:57

Mathematics, 16.06.2020 22:57




Mathematics, 16.06.2020 22:57

Mathematics, 16.06.2020 22:57

Biology, 16.06.2020 22:57


English, 16.06.2020 22:57

Mathematics, 16.06.2020 22:57


English, 16.06.2020 22:57

Mathematics, 16.06.2020 22:57