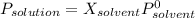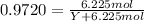Chemistry, 05.05.2020 04:23 NylaJohn29
The common laboratory solvent ethanol is often used to purify substances dissolved in it. The vapor pressure of ethanol , CH3CH2OH, is 54.68 mm Hg at 25 °C. In a laboratory experiment, students synthesized a new compound and found that when 32.83 grams of the compound were dissolved in 286.8 grams of ethanol, the vapor pressure of the solution was 53.15 mm Hg. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight of this compound? ethanol = CH3CH2OH = 46.07 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
The common laboratory solvent ethanol is often used to purify substances dissolved in it. The vapor...
Questions







History, 22.06.2019 23:30