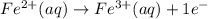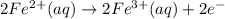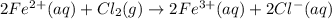Chemistry, 05.05.2020 04:06 milkshakegrande101
What is the balanced redox equation for the voltaic cell? Answer is A) 2 Fe2+(aq) + Cl2(g) \rightarrow → 2 Fe3+(aq) + 2 Cl−(aq)B) 2 Fe3+(aq) + 2 Cl−(aq) \rightarrow → 2 Fe2+(aq) + Cl2(g)C) Fe2+(aq) + Cl2(g) \rightarrow → Fe3+(aq) + Cl−(aq)D) Fe3+(aq) + Cl−(aq) \rightarrow → Fe2+(aq) + Cl2(g)E) None of the above is correct, because neither C(s) not Pt(s) appears in any of them.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
What is the balanced redox equation for the voltaic cell? Answer is A) 2 Fe2+(aq) + Cl2(g) \rightarr...
Questions


Social Studies, 21.08.2019 19:50

Health, 21.08.2019 19:50


Physics, 21.08.2019 19:50








Mathematics, 21.08.2019 19:50





Biology, 21.08.2019 19:50

History, 21.08.2019 19:50