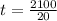Chemistry, 05.05.2020 06:35 genyjoannerubiera
Zinc is to be electroplated onto both sides of an iron sheet that is 20 cm2 as a galvanized sacrificial anode. It is desired to electroplate the zinc to a thickness of 0.025 mm. It is found that a current of 20 A produces a zinc coating of sufficient quality for galvanized iron. Determine the time required to produce the desired coating, assuming 100 % efficiency.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
Zinc is to be electroplated onto both sides of an iron sheet that is 20 cm2 as a galvanized sacrific...
Questions

English, 12.12.2020 16:40









Mathematics, 12.12.2020 16:40

Health, 12.12.2020 16:40



Mathematics, 12.12.2020 16:40


Computers and Technology, 12.12.2020 16:40


Arts, 12.12.2020 16:40

Advanced Placement (AP), 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

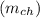 is given as :
is given as :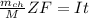
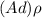 ; replacing that into above equation; we have:
; replacing that into above equation; we have: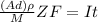 ---- equation (1)
---- equation (1)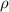 = density
= density ![[ \frac{20 cm^2 *0.0025 cm*7.13g/cm^3}{65.38g/mol}*2 \frac{moles\ of \ electrons}{mole \ of \ Zn} * 9.65*10^4 \frac{C}{mole \ of \ electrons } ] = (20 A) t](/tpl/images/0634/2762/37add.png)