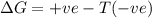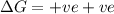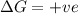Chemistry, 05.05.2020 06:34 Ryleetarver
Use the reaction data in the table below to select the answer choice that best describes this reaction.
Reaction Enthalpy Change
345.7 kJ/mol
Reaction Entropy Change
-25. 3 J/molK
This reaction is never spontaneous.
This reaction is spontaneous at all temperatures.
This reaction is spontaneous at low temperatures.
This reaction is spontaneous at high temperatures

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

You know the right answer?
Use the reaction data in the table below to select the answer choice that best describes this reacti...
Questions


Mathematics, 08.06.2020 07:57

Geography, 08.06.2020 07:57

History, 08.06.2020 07:57

Geography, 08.06.2020 07:57








Mathematics, 08.06.2020 08:57


Mathematics, 08.06.2020 08:57


Mathematics, 08.06.2020 08:57

Mathematics, 08.06.2020 08:57

Spanish, 08.06.2020 08:57

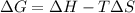
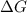 = Gibb's free energy change
= Gibb's free energy change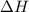 = enthalpy change
= enthalpy change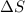 = entropy change
= entropy change