
Chemistry, 05.05.2020 06:28 ricksterv5000
Calcium carbide reacts with water to produce acetylene gas according to the following equation: CaC2(s) + 2H2O(l)C2H2(g) + Ca(OH)2(aq) The product gas, C2H2, is collected over water at a temperature of 25 °C and a pressure of 749 mm Hg. If the wet C2H2 gas formed occupies a volume of 5.87 L, the number of moles of CaC2 reacted was mol. The vapor pressure of water is 23.8 mm Hg at 25 °C. Submit Answer

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 15:30
Acontainer holds 6.4 moles of gas. hydrogen gas makes up 25% of the total moles in the container. if the total pressure is 1.24atm. what is the partial pressure of hydrogen
Answers: 3

Chemistry, 23.06.2019 16:00
Water is called the universal solvent because more substances dissolve in water than in any other chemical. this has to do with the polarity of each water molecule. the hydrogen side of each water (h2o) molecule carries a slight positive electric charge, while the oxygen side carries a slight negative electric charge.
Answers: 3
You know the right answer?
Calcium carbide reacts with water to produce acetylene gas according to the following equation: CaC2...
Questions



Mathematics, 18.03.2021 03:10



Spanish, 18.03.2021 03:10


Mathematics, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10

Arts, 18.03.2021 03:10

Spanish, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10






Mathematics, 18.03.2021 03:10


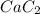 reacted was 0.229
reacted was 0.229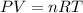
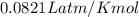
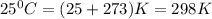
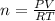
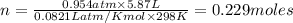
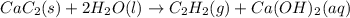
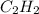 is produced by = 1 mole of
is produced by = 1 mole of 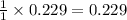 moles of
moles of 

