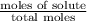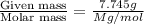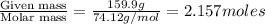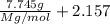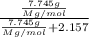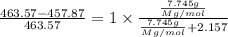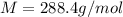The common laboratory solvent diethyl ether (ether) is often used to purify substances dissolved in it. The vapor pressure of diethyl ether , CH3CH2OCH2CH3, is 463.57 mm Hg at 25 °C. In a laboratory experiment, students synthesized a new compound and found that when 7.745 grams of the compound were dissolved in 159.9 grams of diethyl ether, the vapor pressure of the solution was 457.87 mm Hg. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight of this compound?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

You know the right answer?
The common laboratory solvent diethyl ether (ether) is often used to purify substances dissolved in...
Questions



Mathematics, 23.10.2019 23:20


Mathematics, 23.10.2019 23:20

Mathematics, 23.10.2019 23:20




History, 23.10.2019 23:20


History, 23.10.2019 23:20

Social Studies, 23.10.2019 23:20




Mathematics, 23.10.2019 23:20



History, 23.10.2019 23:20

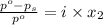
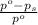 = relative lowering in vapor pressure
= relative lowering in vapor pressure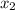 = mole fraction of solute =
= mole fraction of solute =