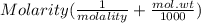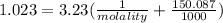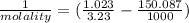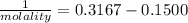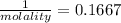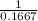An aqueous solution is 3.23M in tartaric acid (C4H06). The solution's density is 1.023 g/mL.
C...

Chemistry, 05.05.2020 09:09 biggiecheese93
An aqueous solution is 3.23M in tartaric acid (C4H06). The solution's density is 1.023 g/mL.
Calculate the solution's molality in tartaric acid.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
Questions

Mathematics, 10.07.2019 20:40


Health, 10.07.2019 20:40


Computers and Technology, 10.07.2019 20:40


Spanish, 10.07.2019 20:40

History, 10.07.2019 20:40


English, 10.07.2019 20:40


English, 10.07.2019 20:40



Mathematics, 10.07.2019 20:40



Mathematics, 10.07.2019 20:40